
Biomedical Engineering Pioneers:
Charles Lindbergh and the Development of the Carrel-Lindbergh Perfusion Pump
By CDR John W. Nelson, MC, USN
 |
On 23 September 1966, LT Vernon Perry, Director of the Tissue Bank of the Navy Medical Research Institute, Bethesda, MD, wrote with excitement to the designer of a unique pump, an engineering and biomechanical innovation. It was a pump that allowed organs to remain "alive" outside the body. In LT Perry's words:
Three weeks ago . . . we managed to get a . . . heart from a monkey . . . and placed it in the pump. We were interested to see if a pH change could be observed in the media after prolonged perfusion. You can imagine our surprise when after one hour of perfusion at room temperature; the heart began to beat independent of the pulsation of your pump. I don't mean that the heart merely fibrillated; there were strong synchronous auricular ventricular contractions. The heart continued to beat for six hours . . . (1)
The recipient of the letter, Charles A. Lindbergh was pleased, but not surprised by the report. The idea for the Carrel-Lindbergh perfusion pump was first conceived in the late 1920=s and completed in the early 1930=s. By the time Lindbergh received Perry=s correspondence, the pump had been used successfully in thousands of experiments where sterile conditions and fine control of physiological operating parameters were essential for tissue and whole organ perfusion.
As was the case with most of his pursuits, the genesis of Lindbergh's interest in biomedical research can be found in personal challenge. In 1929 his sister-in-law was diagnosed with rheumatic heart disease, a disease that carried with it a poor prognosis due primarily to an inability to perform surgical procedures on a beating heart. Once Lindbergh learned that the lack of the surgeon=s ability to provide artificial mechanical means of circulating oxygenated blood prevented a cure, he Amade up his mind to design a pump capable of circulating blood through the body while the heart was being repaired.@(2) Lindbergh enjoyed a reputation as a talented biologist due, in large part, to his work with the United States Department of Agriculture on spore and bacteria surveys of North America, but he had no medical training whatsoever. He studied engineering briefly at the University of Wisconsin, but became disillusioned with "the limits" of formal engineering education and left school prior to completion of his degree, remaining "unencumbered by the accumulated school wisdom that might have discouraged him from the very onset."(2) Armed with his ideas, an innovative mind, and spirit of adventure, Lindbergh pursued his goal of designing and building a mechanical heart/lung machine. For more than 100 years, physiologists had tried to maintain organs alive outside the body with no real success. French physician, scientist and philosopher Julien-Jean-Cesar Legallois (1770-1814) predicted: "If one could substitute for the heart some kind of injection . . . of arterial blood, either natural or artificially made . . . one could succeed easily in maintaining alive indefinitely any part of the body.@(3) Knowing this, Lindbergh presented his concept to a number of physician acquaintances, one of whom arranged a meeting with Dr. Alexis Carrel of the Rockefeller Institute. Lindbergh knew of and respected Carrel whose research emphasized blood vessel suture techniques (for which he was awarded the 1912 Nobel Prize in Medicine), and the culture of cells. Carrel was a pioneer in tissue culture research and wrote prolifically on the subject from the early 1920s. While Carrel's work in the culturing of cells had been ground breaking, he was unable to proceed into the areas of tissue and whole organ culture. He was keenly aware of the technical problems associated with organ perfusion in general, and with cardiopulmonary bypass in particular, most notably the need to add oxygen into the perfusate, a problem finally solved in 1953 by Dr. John Gibbons, the first to use such a bypass system successfully on a patient.(2)
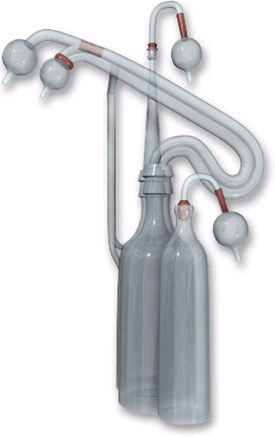 One of Charles Lindbergh's glass perfusion pumps |
With this lack of success as a background, Carrel received Lindbergh's idea with interest, if not for its originality of concept, then certainly because of Lindbergh's record of results in other fields. While he believed that Lindbergh offered a unique engineering approach to the problem, Carrel also appreciated the public relations potential of a collaboration with Lindbergh. Surely, publicity associated with Lindbergh could help assure the continuation of his research and enhance his reputation as a scientist. One is left to wonder, for example, if Carrel would have appeared on the 13 July 1938 cover of TIME Magazine were it not for Lindbergh=s popularity as a national hero. Charles Lindbergh=s stature as a public figure during this time in American history cannot be overstated. Indeed Lindbergh himself did not fully appreciate the magnitude and strength of his public appeal and popularity, popularity that he ironically tried to avoid from the time of his famous flight until his death in 1974. Many of Carrel's colleagues at the Rockefeller Institute privately questioned the scientific value of Lindbergh's contribution when their collaboration was first announced. "Some of the senior members were inclined to disapprove of the introduction of an amateur to the select ranks of medical investigator; others feared sensational publicity."(6) Others were openly critical, denouncing the partnership as a publicity stunt, rather than a scientific collaboration. However, Lindbergh carried out his work with Amodesty and discretion,@ publishing his early findings anonymously. In fact, no public announcement of Lindbergh's presence at the institute was made until mid-1935.
Carrel's previous experience in the field of cell profusion revealed the overly ambitious nature of Lindbergh's original plan.(7) He convinced the inventor that, instead of venturing immediately into a difficult and unexplored field of heart lung bypass, "it was wiser to attempt the culture of whole organs, which could become an almost immediate reality . . . ."(2) He knew that, whether or not the treatment of diseased human organs by exchange or replacement ever became possible, "the really important application of the method would not be in the field of surgery, but in physiology . . . ,@(6) a tool to fulfill Carrel's wish to Astudy the interplay between organ, blood, and lymph.@(2)
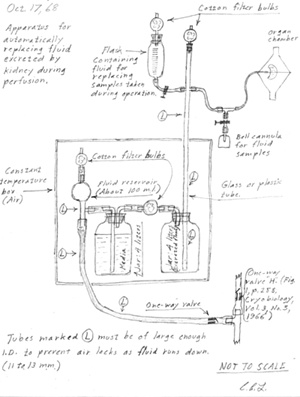 Lindbergh's drawing of his perfusion pump dated October 17, 1968. Click on the image to enlarge. |
Based on the success of their initial collaboration, Lindbergh and Carrel undertook an ambitious project: the perfusion of whole organs. As Lindbergh wrote in 1965, their plan was set to proceed in three stages: AFirst, the development of a pulsating perfusion pump that would approximately duplicate natural pressures, and in which infection could be excluded. Second, the development of surgical and chemical techniques related to installation of the organ and the perfusing fluid. Third, the application of the method to research projects.@(9)
Lindbergh's major contribution was introduced in a paper published jointly with Carrel in which he describes an all-glass system for the perfusion of whole organs. It is in the design and manufacture of this device that Lindbergh's skills as a biomedical engineer are best demonstrated. Based on lessons learned from previous versions, and using the diverse talents of Rockefeller Institute colleagues, he was able to deliver an apparatus that met all of Carrel's strict criteria. It was ultimately used, as Lindbergh recalled, in "over a thousand perfusion experiments.@(9) Lindbergh=s design provided careful environmental control, allowed researchers to add or remove tissue and perfusion fluid from the system without interrupting operations, allowed microscopic viewing of the tissues in vitro, and provided an aseptic environment. A working model was first delivered in 1935, followed in 1938 with the publication of The Culture of Organs, a work designed to serve as a step-by-step technical manual for fellow researchers. In it, Lindbergh explains that the apparatus . . . Amaintains a sterile pulsating circulation through the [living] organs for a length of time limited only by the condition of the organ and the perfusion fluid.@(10)
Thanks to the technical skill of Rockefeller Institute=s glass blowers, Lindbergh designed and built the system entirely of Pyrex glass with rubber stoppers and cotton filters, all with anti-sepsis and ease of cleaning in mind. The system was operated entirely using compressed control gas pressure (oxygen, carbon dioxide, and nitrogen) as a motive force to provide pulsating fluid at adjustable pressure and measurable flow rate. Maintenance of system pressure within strict parameters while allowing the introduction of new profusion fluid and/or additional specimens was a difficult challenge, yet the originality of Lindbergh's approach exceeded expectations.
Lindbergh's ingenious design required seventeen pages of detailed descriptive text and seven full-page illustrations to adequately describe, in part explaining that the device A. . . has only three openings that communicate with the exterior. These openings are protected against infection by filter bulbs containing non-absorbent cotton. Neither the organ nor the perfusion fluid comes in contact with any stoppers or joints which communicate with the exterior . . .. The composition of all gas in contact with the organ and the perfusion fluid is controlled. Foaming and evaporation of the fluid are prevented. The maximum and minimum pulsation pressures and the pulsation rate are adjustable. The pressure at various points in the pulse cycle can be controlled. The temperature of operation is adjustable. The rate of flow of perfusion fluid can be measured. Changes for rate of flow through the organ are compensated for automatically with a minimum effect on pulsation pressures. The perfusion fluid is filtered during its circulation and before it enters the organ. Organs can be removed from one apparatus and installed in another aseptically. The perfusion fluid can be removed and replaced aseptically. The organ and the perfusion fluid can be observed at all times.(11)
 L to R: CDR G.H. Mouer, LT V.P. Perry, C.A. Lindbergh, and T. Malinin view a model of the pump. Click on the image to enlarge. |
In the months following Copenhagen, American and European labs ordered dozens of Lindbergh pumps, but for various reasons they were not widely used. One reason was a shift within the scientific community toward study at the level of individual cells and away from whole organs and organ systems. Additionally, biochemists found that they could obtain as satisfactory a result from cut sections of organs (which remained viable for a few hours after sectioning) as they could from whole, perfused organs. However, the main reason for the failure of the Lindbergh pump to gain wide use within the scientific community was its difficulty of operation. As a result, virtually all the Lindbergh pumps constructed between 1935 and 1938 had dropped out of use by 1940.(6)
Lindbergh continued to work with Carrel to improve the profusion system, including the pump, culture medium, and perfusion fluid, until the early 1940's. Of the original three-step plan previously introduced, Lindbergh wrote with a hint of disappointment, . . . Awe had completed (with reasonable satisfaction for preliminary work) the first two stages. The war and Carrel's death prevented our entering the third. Of course, even in the first two stages much additional development was desirable.@(13)
While organ profusion, with an eye toward organ transplant, continued to develop within the scientific community after the war, Lindbergh's own active pursuit of further study in the subject ended until persuaded to return to it some 30 years later.
During the 1960's, researchers at the Navy Medical Research Institute (NMRI) Tissue Bank in Bethesda, MD, carried out a series of studies designed to examine the preservation of whole organs, possibly through (then) new freeze-dried technology, for use in transplant at field medical facilities. Based on research performed on skin, bone marrow, and blood, NMRI scientists had concluded that it was possible to freeze-dry and store some tissue grafts for over 10 years, while remaining clinically viable.(14) However, work on whole organs presented many daunting problems. Tissue Bank scientists studied all existing research in whole organ perfusion, including that of Lindbergh and Carrel (then held by the Georgetown University Medical Center) and found that they had reported better results than those attained using more recent techniques.(15)
The original perfusion pump described by Lindbergh required only minor modifications to work properly at temperatures required for freeze-drying. LT Vernon P. Perry (Director, NMRI Tissue Bank) encouraged Lindbergh to come out of retirement and participate in a collaborative effort toward a pump redesign. Initially, Lindbergh was reluctant, writing from Switzerland in 1965 that A. . . it has been so many years since I have done any lab work in connection with tissue or organ culture that I would have very little to contribute. Although my interests naturally continue in these fields, my last active research dates back to about 1938.@(16)
After repeated requests, Lindbergh finally agreed, and in 1968 accepted an appointment as a guest scientist at NMRI to resume work on whole organ perfusion. The collaboration produced two publications, AAn apparatus for the pulsating perfusion of whole organs" (17) and "Maintenance of Continuous Contraction of Mammalian Hearts at Hypothermic Temperatures" (18) but ended shortly thereafter when NMRI abandoned their original plan.
It is interesting to note that following the 1936 Copenhagen perfusion pump demonstration, conventional wisdom held that "Lindbergh's work as a scientist would probably be remembered long after his flight to Paris is only dimly recalled event in aviation history.@(12) While this has certainly not been the case, Charles Lindbergh's contribution to our body of scientific knowledge is remarkably noteworthy, if not for its lasting benefit to medical research, then for the pioneering, innovative spirit it represents.
References
- Perry VP to Lindbergh CA, 23 Sep 1966. C. A. Lindbergh correspondence, Naval Medical Research Center Archives, Bethesda, MD.
- Bing R. Lindbergh and the biological sciences (a personal reminiscence). Texas Heart Institute Journal. 1987;14:231-237.
- "Glass Heart," Time. 1 Jul 1935:41-42.
- Carrel A. A method for the physiological study of tissues in vitro. J of Exp Med. 1923;38:407-418.
- Witkowski JA. Alexis carrel and the mysticism of tissue culture. Medical History. 1979;23:279-296.
- Corner GW. A History of the Rockefeller Institute. The Rockefeller Institute Press, New York, NY. 1964. pp. 230, 232, 235.
- Schumaker HB. The Evolution of Cardiac Surgery. Indiana University Press, Bloomington, IN. 1992. p. 248
- Perry VP. Charles a. Lindbergh: 1902-1974. In Vitro. 1975;11(5):247-250.
- Lindbergh CA to Perry VP, 9 Apr 1965. C.A. Lindbergh correspondence, Naval Medical Research Center Archives, Bethesda, MD.
- Carrel A and Lindbergh CA. The culture of whole organs. Science. 1935;81(2112):621-623.
- Carrel A and Lindbergh CA. The Culture of Organs. Paul B. Hoeber, Inc, New York, NY. 1938. p.16
- "Lindbergh Adds To Fame As Scientist," The Literary Digest. 22 Aug 1936:16-17.
- Lindbergh CA to Perry VP, 9 Apr 1965. C. A. Lindbergh correspondence, Naval Medical Research Center Archives, Bethesda, MD.
- Gresham RB, et. al. Report on Current Methods and Experimental Approaches to the Future. J Am Med Assn. 1963;183:13-14.
- "Guest Investigator at the Tissue Bank of the Naval Research Institute, Bethesda," New York Herald Tribune-Washington Post. Mar 15, 1967;p 5.
- Lindbergh CA to Perry VP, 27 Sep 1965. C. A. Lindbergh correspondence, Naval Medical Research Center Archives, Bethesda, MD.
- Lindbergh CA, Perry VP, et. al. An apparatus for the pulsating perfusion of whole organs. Cryobiology. Nov-Dec 1966;3(3):252-60.
- In Memoriam: Vernon P. Perry, LCDR MSC USN (RET) (1927-1990). Cryobiology. 1991;28:207-209.
Reprinted with permission from Dr. Nelson & the Naval Medical Research Center
Navy Medicine Magazine, Bureau of Medicine and Surgery (M09H), Washington, DC, and Dr. Nelson.
Privacy Policy | Terms and Conditions | This site is not affiliated with the Lindbergh family,
Lindbergh Foundation, or any other organization or group.
This site owned and operated by the Spirit of St. Louis 2 Project.
Email: webmaster@charleslindbergh.com
® Copyright 2014 CharlesLindbergh.com®, All rights reserved.
Help support this site, order your www.Amazon.com materials through this link.

